Литература
1. Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–2650.
2. Lundy JS. Balanced anesthesia. Minn Med. 1926;9:399–404.
3. Hendrickx JF, Eger EI II, Sonner JM, Shafer SL. Is synergy the rule? A review of anesthetic interactions producing hypnosis and immobility. Anesth Analg. 2008;107:494–506.
4. Purves D, Augustine GJ, Fitzpatrick D, Hall WC, Lamantia A, White LE. Purves D, Augustine GJ, Fitzpatrick D, Hall WC, Lamantia A, White LE. Pain. In: Neuroscience. 2012:5th ed. Sunderland, MA: Sinauer Associates, Inc; 209–228.
5. Lake APJ. Balanced anaesthesia 2005: avoiding the transition from acute to chronic pain. South Afr J Anaesth Analg. 2005;11:14–18.
6. McNicol E, Horowicz-Mehler N, Fisk RA, et al.; American Pain Society. Management of opioid side effects in cancer-related and chronic noncancer pain: a systematic review. J Pain. 2003;4:231–256.
7. Volkow ND, Collins FS. The role of science in the opioid crisis. N Engl J Med. 2017;377:1798.
8. Brown EN, Purdon PL, Van Dort CJ. General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu Rev Neurosci. 2011;34:601–628.
9. Dunn LK, Durieux ME. Perioperative use of intravenous lidocaine. Anesthesiology. 2017;126:729–737.
10. Mulier J. Opioid free general anesthesia: a paradigm shift? Rev Esp Anestesiol Reanim. 2017;64:427–430.
11. Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474.
12. Rabiner EA, Beaver J, Makwana A. Pharmacological differentiation of opioid receptor antagonists by molecular and functional imaging of target occupancy and food reward-related brain activation in humans. Mol Psychiatry. 2011;16:826–835.
13. Burn DJ, Rinne JO, Quinn NP, Lees AJ, Marsden CD, Brooks DJ. Striatal opioid receptor binding in Parkinson’s disease, striatonigral degeneration and Steele-Richardson-Olszewski syndrome, A [11C]diprenorphine PET study. Brain. 1995;118(pt 4):951–958.
14. Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem. 2004;73:953–990.
15. Stein C. The control of pain in peripheral tissue by opioids. N Engl J Med. 1995;332:1685–1690.
16. Fukuda K. Opioids. 2009.7th ed. New York, NY: Churchill Livingstone.
17. Veinante P, Yalcin I, Barrot M. The amygdala between sensation and affect: a role in pain. J Mol Psychiatry. 2013;1:9.
18. Becerra L, Harter K, Gonzalez RG, Borsook D. Functional magnetic resonance imaging measures of the effects of morphine on central nervous system circuitry in opioid-naive healthy volunteers. Anesth Analg. 2006;103:208–216.
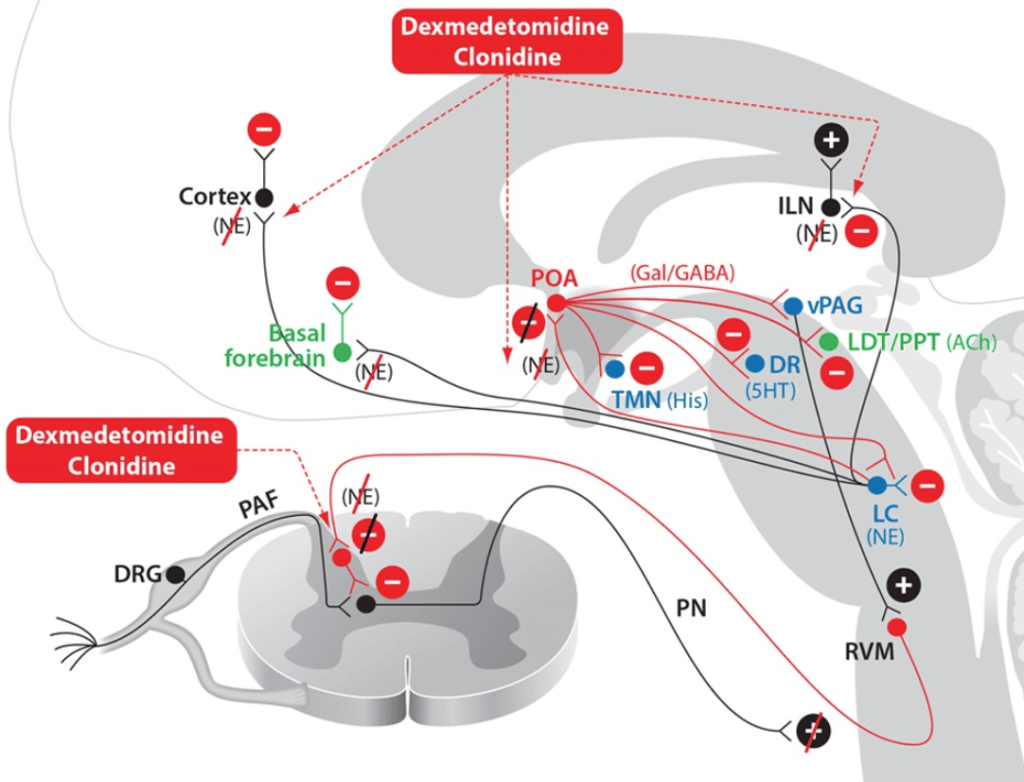
19. Lydic R, Baghdoyan HA. Sleep, anesthesiology, and the neurobiology of arousal state control. Anesthesiology. 2005;103:1268–1295.
20. Mortazavi S, Thompson J, Baghdoyan HA, Lydic R. Fentanyl and morphine, but not remifentanil, inhibit acetylcholine release in pontine regions modulating arousal. Anesthesiology. 1999;90:1070–1077.
21. Griffioen KJ, Venkatesan P, Huang ZG. Fentanyl inhibits GABAergic neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Brain Res. 2004;1007:109–115.
22. Sinner B, Graf BM. Ketamine. Handb Exp Pharmacol. 2008:313–333.
23. Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–533.
24. Seamans J. Losing inhibition with ketamine. Nat Chem Biol. 2008;4:91–93.
25. Purdon PL, Sampson A, Pavone KJ, Brown EN. Clinical electroencephalography for anesthesiologists: part I: background and basic signatures. Anesthesiology. 2015;123:937–960.
26. Boon JA, Milsom WK. NMDA receptor-mediated processes in the parabrachial/Kölliker fuse complex influence respiratory responses directly and indirectly via changes in cortical activation state. Respir Physiol Neurobiol. 2008;162:63–72.
27. Fuller PM, Fuller P, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519:933–956.
28. Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Research. 1984;319:229–259.
29. Akeju O, Song AH, Hamilos AE. Electroencephalogram signatures of ketamine anesthesia-induced unconsciousness. Clin Neurophysiol. 2016;127:2414–2422.
30. Do SH. Magnesium: a versatile drug for anesthesiologists. Korean J Anesthesiol. 2013;65:4–8.
31. Pairu J, Triveni GS, Manohar A. The study of serum calcium and serum magnesium in pregnancy induced hypertension and normal pregnancy. Int J Reprod Contracept Obstet Gynecol. 2015;4:30–34.
32. Gourgoulianis KI, Chatziparasidis G, Chatziefthimiou A, Molyvdas PA. Magnesium as a relaxing factor of airway smooth muscles. J Aerosol Med. 2001;14:301–307.
33. Ruppersberg JP, Kitzing E, Schoepfer R. The mechanism of magnesium block of NMDA receptors. Semin Neurosci. 1994;6:87–96.
34. Seyhan TO, Tugrul M, Sungur MO. Effects of three different dose regimens of magnesium on propofol requirements, haemodynamic variables and postoperative pain relief in gynaecological surgery. Br J Anaesth. 2006;96:247–252.
35. Andrieu G, Roth B, Ousmane L. The efficacy of intrathecal morphine with or without clonidine for postoperative analgesia after radical prostatectomy. Anesth Analg. 2009;108:1954–1957.
36. Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263.
37. Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18:4705–4721.
38. Akeju O, Kim SE, Vazquez R. Spatiotemporal dynamics of dexmedetomidine-induced electroencephalogram oscillations. PLoS One. 2016;11:e0163431.
39. Akeju O, Pavone KJ, Westover MB. A comparison of propofol- and dexmedetomidine-induced electroencephalogram dynamics using spectral and coherence analysis. Anesthesiology. 2014;121:978–989.
40. Bautmans I, Njemini R, De Backer J, De Waele E, Mets T. Surgery-induced inflammation in relation to age, muscle endurance, and self-perceived fatigue. J Gerontol A Biol Sci Med Sci. 2010;65:266–273.
41. Arias J, Aller M-A, Arias J-I. Surgical Inflammation. 2013.Madrid, Spain: Bentham Science Publishers.
42. Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000.
43. Ajmone-Cat MA, Bernardo A, Greco A, Minghetti L. Non-steroidal anti-inflammatory drugs and brain inflammation: effects on microglial functions. Pharmaceuticals (Basel). 2010;3:1949–1965.
44. Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235.
45. Berde C, Strichartz GR. Miller R, Eriksson L, Fleisher L, Wiener-Kronish J, Cohen N, Young W. Local Anesthetics. In: Miller’s Anesthesia. 2015:8th ed. Philadelphia, PA: Elsevier; 1028–1053.
46. Wang GK, Strichartz GR. State-dependent inhibition of sodium channels by local anesthetics: a 40-year evolution. Biochem (Mosc) Suppl Ser A Membr Cell Biol. 2012;6:120–127.
47. Hollmann MW, Herroeder S, Kurz KS. Time-dependent inhibition of G protein-coupled receptor signaling by local anesthetics. Anesthesiology. 2004;100:852–860.
48. Miralda I, Uriarte SM, McLeish KR. Multiple phenotypic changes define neutrophil priming. Front Cell Infect Microbiol. 2017;7:217.
49. Hollmann MW, McIntire WE, Garrison JC, Durieux ME. Inhibition of mammalian Gq protein function by local anesthetics. Anesthesiology. 2002;97:1451–1457.
50. Wagman IH, De Jong RH, Prince DA. Effects of lidocaine on the central nervous system. Anesthesiology. 1967;28:155–172.
51. Muth-Selbach U, Hermanns H, Stegmann JU. Antinociceptive effects of systemic lidocaine: involvement of the spinal glycinergic system. Eur J Pharmacol. 2009;613:68–73.
52. Cummins TR, Sheets PL, Waxman SG. The roles of sodium channels in nociception: implications for mechanisms of pain. Pain. 2007;131:243–257.
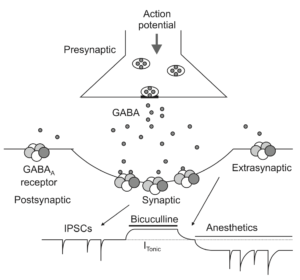
53. Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987;20:365–383.
54. Hemmings HC Jr, Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26:503–510.
55. Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–386.
56. Purdon PL, Pierce ET, Mukamel EA, et al. Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proc Natl Acad Sci U S A. 2013;110:E1142–E1151.
57. Lewis LD, Weiner VS, Mukamel EA, et al. Rapid fragmentation of neuronal networks at the onset of propofol-induced unconsciousness. Proc Natl Acad Sci U S A. 2012;109:E3377–E3386.
58. Ching S, Cimenser A, Purdon PL, Brown EN, Kopell NJ. Thalamocortical model for a propofol-induced alpha-rhythm associated with loss of consciousness. Proc Natl Acad Sci U S A. 2010;107:22665–22670.
59. Flores FJ, Hartnack KE, Fath AB, et al. Thalamocortical synchronization during induction and emergence from propofol-induced unconsciousness. Proc Natl Acad Sci U S A. 2017;114:E6660–E6668.
60. Dolosys GmbH. The Dolosys Paintracker. 2017. Available at: http://www.dolosys.de/Products-EN.htm. Accessed June 29, 2017.
61. ANI (Analgesia Nociception Index). Available at: https://www.mdoloris.com/en/technologies/ani-analgesia-nociception-index/. Accessed August 19, 2018.
62. Storm H. Med-Storm. 2016.PainMonitor™:Oslo, Norway.
63. Huiku M, Kamppari L, Viertio-Oja H. Surgical Plethysmographic Index (SPI) in Anesthesia Practice. 2014.Helsinki, Finland: General Electric Healthcare.
64. Brown EN, Solt K, Purdon PL, Akeju O. Miller R, Eriksson L, Fleisher L, Wiener-Kronish J, Cohen N, Young W. Monitoring brain state during general anesthesia and sedation. In: Miller’s Anesthesia. 2015:8th ed. Philadelphia, PA: Elsevier; 1524–1540.
65. Clarke R, Derry S, Moore RA. Single dose oral etoricoxib for acute postoperative pain in adults. Cochrane Database Syst Rev. 2014:CD004309.
66. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152:292–298.
67. Kumar K, Kirksey MA, Duong S, Wu CL. A review of opioid-sparing modalities in perioperative pain management: methods to decrease opioid use postoperatively. Anesth Analg. 2017;125:1749–1760.